Wound infections don't usually enter the blood and become systemic, spreading the infection throughout our bodies, and there's a good reason for that: Our bodies actively work to prevent it, according to research that discovered a new use for a protein first discovered decades ago.
A collaborative group of researchers from Singapore, Copenhagen, and Lund University in Sweden has figured out the protective mechanism our bodies use to contain infections at their source, and it involves a protein we know very well. Thrombin is an enzyme in the blood that's critical to the clotting process, and it also rounds up bacteria and toxins to prevent them from traveling to other parts of the body and setting up an infection there, as it turns out.
The new findings, by lead author Jitka Petrlova from Lund University, along with his colleagues, was published online in the journal Proceedings of the National Academy of Sciences.
An Old Role & New Role
Thrombin has a well-established role in clotting. It's an enzyme that creates the blob we know as a clot. The process stops bleeding, plugs broken vessels, and protects wounds.
Scientists knew thrombin was present in wounds, but they found another purpose for it there. When white blood cells called neutrophils rush to the scene of an infection, they release an enzyme called elastase that cuts thrombin into pieces.
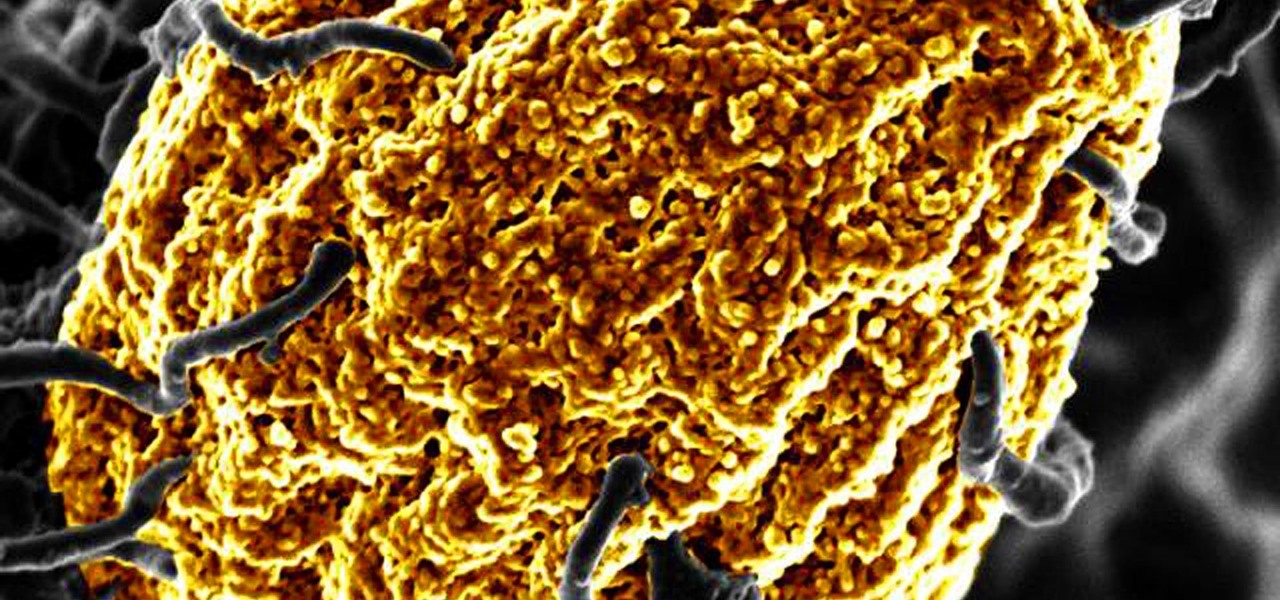
So, the researchers cut thrombin into pieces in the lab to mimic what happens at a wound site. When they mixed the thrombin pieces with E. coli bacteria or lipopolysaccharide (a toxin the bacteria produce) in a test tube, the thrombin pieces caused the bacteria and toxin to aggregate quickly into digestible clumps.
Using several sophisticated techniques, including electron microscopy and fluorescent staining of bacteria so it could be more easily visualized, the scientists tracked what happened when they added E. coli to cells and fluid collected from human wounds. Once the enzyme contained the bacteria within an aggregate, body's macrophages could digest the clumped pathogens, effectively stopping the infection.
Amyloids are deposits of proteins, and the team found that thrombin formed amyloid deposits in the process of aggregating bacteria and toxins. Amyloid aggregates are observed and may be part of the disease process in Parkinson's, Huntington's, and Alzheimer's disease.
The research team suggested that the thrombin response to infection may sometimes go awry:
Our discovery links aggregation and amyloid formation to our primary defense against infections — our innate immunity. It is well known that various aggregating proteins can cause amyloid disease, in skin or internal organs, such as the brain. Therefore, a mechanism that is supposed to protect us from infections can sometimes be over-activated and lead to degenerative diseases.
'New' Defense Is Millions of Years Old
This discovery of a new defense against infections is an example of our innate immune system. We are born with this defense capability, and it kicks in as soon as a foreign pathogen is detected.
The discovery of thrombin's role in the innate immune response may be new, but the researchers believe the process that contains infections at the site of a wound has been at work protecting humans for millions of years. Thrombin's ability to prevent a local wound infection from becoming a systemic infection has been instrumental in the survival of the human race.
Compared to antibiotics, innate immunity has been around for millions of years — and I think we should consider the application of these concepts in an era of increasing antibiotic resistance.
The scientists may have a point. A process that has persisted for millions of years and helped to ensure our evolutionary survival might have a lot to teach us about how to manage infections.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.





















Be the First to Comment
Share Your Thoughts