Scientists are constantly on the search for new organisms, species, and other types of life. A special group of these researchers, calling themselves "bioprospectors," dive deep into mines to find unique lifeforms with special properties not found anywhere else.
Recently, a soil bacterium found by bioprospectors at the University of Kentucky's Center for Pharmaceutical Research and Innovation (CPRI) has yielded an enzyme that can produce more potent versions of drugs. Specifically, this one was used to improve daptomycin—an antibiotic used to treat bacterial infections, including Staphylococcus aureus.
On the Hunt for New Bacteria
One of the places that the CPRI look for unique natural products is the coal fires of eastern Kentucky, and this bacterium came from the the smoke vents of the Ruth Mullins fire. Kentucky coal fires are named after the person who first reported the fire, so in this case, it was Ruth Mullins who smelled the unmistakeable stench of sulfur fumes 10 years ago.
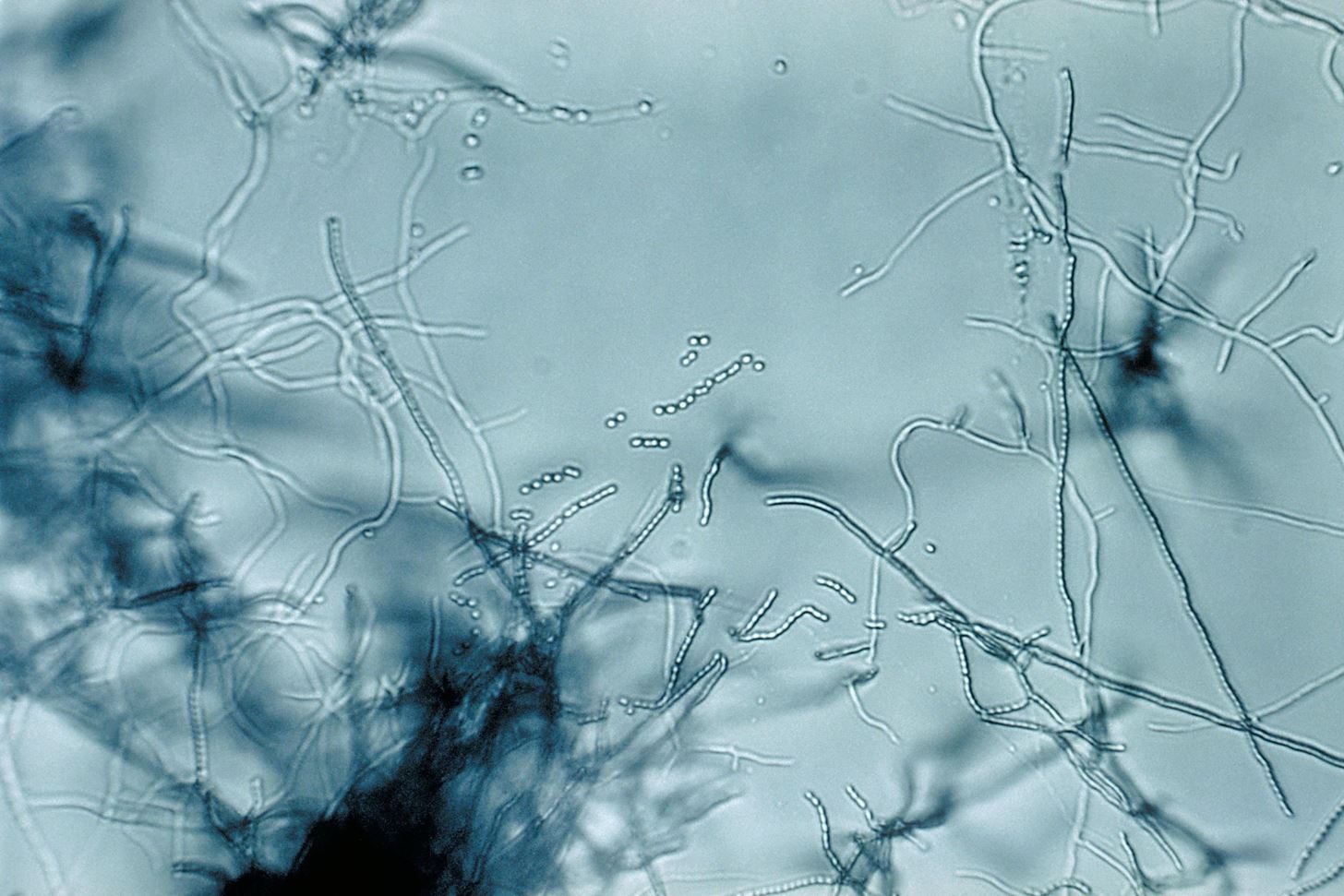
The special coal fire bacteria that was discovered is a type of Streptomyces, which was named RM-5-8, after the area it was found in.
Underground coal fires, like the ones in Kentucky, probably started by lightning or forest fires and keep burning because they have sources of fuel, like coal and other carbon sources, and access points of air through natural vents in the ground.
Each fire site is different and changes all the time as it burns different materials around them. Heated soil, minerals, and tree roots provide good targets for the search for organisms such as unique and diverse bacteria. Bacteria commonly associated with normal soil flora move in when the soil cools back down.
In 2013, the CPRI team, with the help of the Kentucky Geological Survey, drilled a well 4,800 feet deep in an eastern Kentucky coal field, took multiple core samples at different depths, put extracts of the soil on plates of laboratory media, and watched for the bacteria, often very slowly, to grow.
After running biochemical tests to identify them or categorize them, the bacterial data were entered into a repository at the University of Kentucky and made available for study by other researchers. In the year since the repository had been open, 60 new compounds had been entered—like the enzyme used to increase the potency of daptomycin.
The work done to find the enzyme's superpowers was a joint effort from the CPRI and other departments at the University of Kentucky, as well as the Department of Biosciences at Rice University. They published their findings in the journal Nature Chemical Biology.
The bacterial enzyme they found isn't just important for antibiotics. This reaction "could be broadly useful in producing drugs and other chemicals," said co-author and Rice structural biologist George Phillips, in a press release from Rice University. His team determined the three-dimensional structure of the enzyme.
The Newest Bacterium Find, but Not the Last
Streptomyces is found in abundance in soil and decaying vegetation, so it was no surprise to find it in a coal mine. This group of bacteria produce over two-thirds of the biologically-derived antibiotics, such as neomycin, cypemycin, grisemycin, bottromycin, and chloramphenicol—and the one named after them, streptomycin.
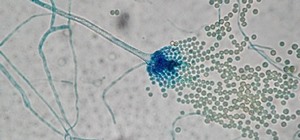
RM-5-8 makes an enzyme called L-tryptophan C6 C-prenyltransferase PriB, which has a unique characteristic: it can be used to make antibiotics work better. Enzymes are proteins that act as a catalyst to facilitate biochemical reactions: They make them happen faster than they would naturally, though they don't change the end products of the reaction.
Specifically, prenyltransferase enzymes, like the one isolated from RM-5-8, modify simple molecules by adding or removing chemical groups called prenyls. These groups are carbon containing structures that help large molecules attach to cells.
But the coal fire enzyme was different. It was a "permissive enzyme," which modifies more complex compounds, like antibiotics. The researchers put it to the test on daptomycin to see if it could modify the drug and make related compounds that can potentially work better than the original.
One of the new daptomycins was 6- to 10-fold more potent in killing bacteria than the current daptomycin, without killing other cells in the body. The researchers aren't sure why the modified antibiotic works better, but speculated that the added prenyl group helps the drug get through bacterial membranes easier, so it can kill the germs better.
According to Jon Thorson, co-author of the study and Director of the CPRI, natural products evolve for a function and we can make a minor changes to them that might lead to dramatic changes in their function or potency. Starting with unique, unusual, and new organisms gives the scientists a leg up because it can be a rapid way to study medicinal chemistry.
Thorson's specialty is to take a molecule and diversity it—change it so that they may have other applications. That's how the new daptomycin was developed and it likely will not be the last new product made possible by the search for unusual new lifeforms by this group of bioprospectors from the CPRI.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.




















Be the First to Comment
Share Your Thoughts