On October 17, 1943, a story in the New York Herald Tribune read "Many laymen — husbands, wives, parents, brothers, sisters, friends — beg Dr. Keefer for penicillin," according to the American Chemical Society. Dr. Chester Keefer of Boston was responsible for rationing the new miracle drug, penicillin.
Fast forward nearly 75 years. Penicillin, our magic bullet, no longer kills the most dangerous bacteria — due to rapidly accelerating rates of antibiotic resistance. Scientists have been in a race against time to find new antibiotics to handle the superbugs, disease-causing bacteria resistant to multiple antibiotics.
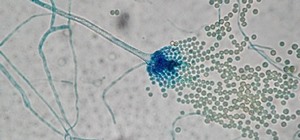
In a case of scientific déjà vu, a research group led by biologist Jens Nielsen at Chalmers University of Technology in Gothenburg, Sweden, have identified over 1,000 genes in penicillin mold with the potential to yield those new antibiotics. Their findings were published online in Nature Microbiology.
To find compounds in the mold that might be useful therapeutically, scientists sequenced the genomes of 24 of 300 different penicillium mold species — nine of which were new species. Then they dove deeper into the genes to identify clusters that might produce useful metabolic products, much like original penicillin produced by a mold in a scientist's lab experiment.
The researchers found a huge number of mold genes — 1,317 — with the potential to yield metabolites we could use. Through their analysis, they could predict that two species not previously known to produce the antifungal agent yanuthone actually did, and they realized the magnitude of what the new gene sequences could tell them.
Now, the team can transplant entire gene clusters into yeast cells to study the compounds they produce — or even mix and match the genes to produce compounds that don't exist in nature. The door to the antibiotic and pharmaceutical possibilities these molds may yield has been blown wide open.
"We're just beginning to scratch the surface," said Nielsen in an interview with Cosmos magazine.
Thanks to Nielsen and his colleagues, penicillin mold has been given a second look, and it appears that the fungus has a lot more to give us than just penicillin.
One Twist of Fate After Another
The discovery of penicillin by Alexander Fleming in 1928 meant that pneumonia, gonorrhea, rheumatic fever, and simple wound infections were no longer death sentences.
Returning from vacation — and his neglected petri dishes in the incubator — he found them growing mold, along with the bacteria he had inoculated them with. The very observant Fleming realized that the bacteria was not growing near the mold, as if the mold had secreted something that inhibited bacterial growth.
In June 1929, Fleming published his findings in the British Journal of Experimental Pathology, but he made only a passing reference to penicillin's therapeutic potential.

Thankfully, Howard Florey, Ernst Chain, and their colleagues at the Sir William Dunn School of Pathology at Oxford University saw the potential and started to purify penicillin from the mold's "juice" — something that the mold exuded into liquid. They set up fermentation vessels to draw out the broth under the moldy surface and employed a team of "penicillin girls" to grow the mold and look after the fermentation.

By 1940, Florey was running experiments to find out exactly what penicillin could do. This crude penicillin mold extract could protect mice against infection from deadly Streptococci bacteria. On February 12, 1941, Albert Alexander, a 43-year-old policeman who had developed a life-threatening infection after scratching himself pruning roses, became the first recipient of the Oxford penicillin. He made a miraculous recovery within days.
Lack of resources needed to produce the Oxford penicillin, coupled with an anticipated steep demand from World War II, led Florey and colleague Norman Heatley to travel to the US to enlist the help of pharmaceutical companies to increase production of the new antibiotic.
Fate intervened again, and Florey and Heatley were referred to Charles Thom of the Department of Agriculture — a prominent mycologist and authority on the Penicillium mold — and eventually to the Department's Northern Regional Research Laboratory in Peoria, Illinois. That relationship made large-scale production of penicillin possible.
Soon, Merck, Pfizer, Squibb, and other companies joined in the production of penicillin, which increased dramatically from 21 billion units in 1943 to 1,663 billion units in 1944 — to more than 6.8 trillion units in 1945.
By March 15, 1945, penicillin was available to the general public at their corner pharmacy. Since that time, researchers estimate that penicillin has saved 200 million lives.
In February 2017, the World Health Organization released a list of the 12 most dangerous superbugs — dangerously pathogenic multi-drug resistant bacteria—and they are all resistant to penicillin. The imminent threats these bacteria pose to global human health is fueling the race to find new antibiotics.
In yet another twist of fate, penicillin mold may yield a product that rescues us. Again.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.




















Be the First to Comment
Share Your Thoughts